
This 2-day intensive in-house course provides comprehensive training in the revised ISO 13485:2016 standard and the including a review of provisions and requirements of ISO 19011, auditing processes and procedures, the follow-up process and more. The "hands-on" program includes lectures, workshops and a case study to increase participant knowledge and learning.
This course is also available for in-house presentation with or without the 13485:2016 certifications. Get best information on A Process Approach Internal Auditing to ISO 13485:2016 methodologies. Also, this training course may be used in its original presentation format or it may be customised by referring to the degree of business nature.
Date: 22th & 23th December 2021
Venue: Polytool Technologies Sdn. Bhd., Bayan Lepas, Penang
Course Objectives:
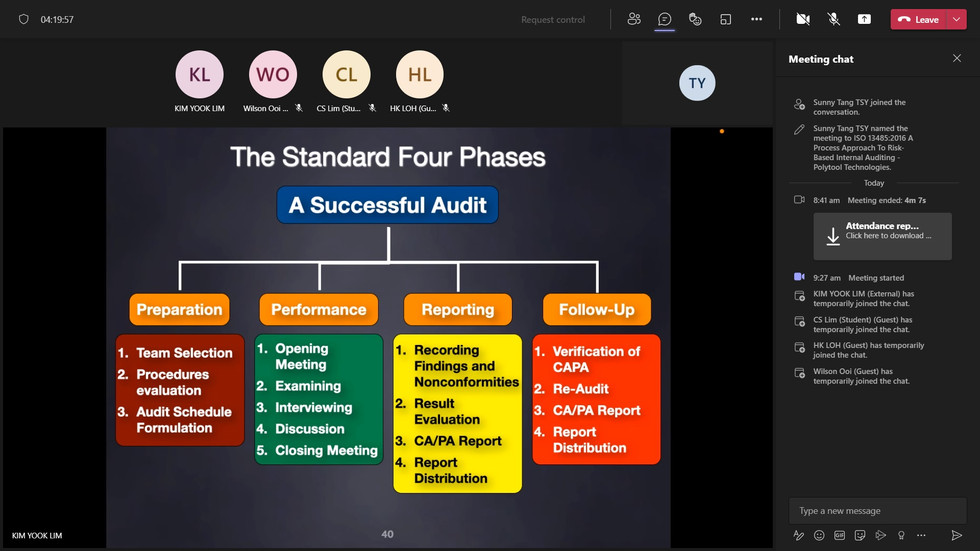
This course meets the essential skills and techniques required by ISO 19011 for internal auditors. Participants will be able to perform the Internal Audit in their audit assignment based on the 4-Phase Internal Auditing Model. Here are the learning objectives for the two days training program; in which participants will be able to:
Relate the applicable ISO 13485:2016 requirements to the organization QMS.
Analyse the applicable ISO 13485:2016 and other requirements, including risks and opportunities for area to be audited
Prepare the audit checklist based on the process mapping and analysis
Carry out the auditing activities based on the process mapping and audit checklist
Apply the internal audit tools and techniques to carryout the internal audit.
Evaluate the significance of positive and negative audit findings.
Who Should Attend:
Quality Managers, RA Managers, Auditors of Medical Device, Executives & Senior Management, Cross functional team members of implementation project, Suppliers to the organisation. Supervisors and Line Leaders.
More Training Pictures:
Feel free to contact TSY Consultancy by Call / WhatsApp to +6012-6243921 or email to marketing@tsy.my
Cheers.













Comments